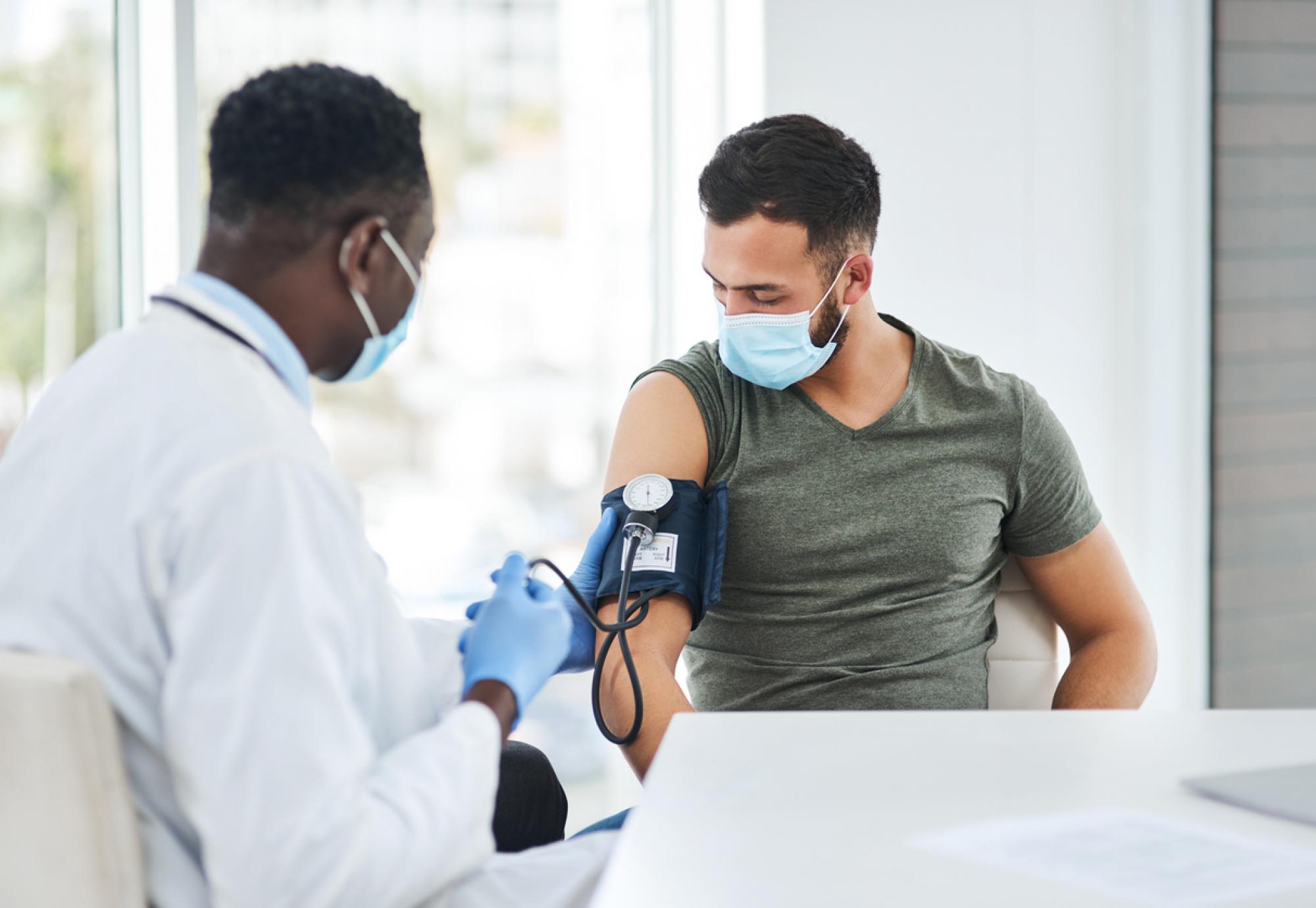
The world’s first ever injection-based treatment for high blood pressure will be given to 100 trial patients over the next three years.
The study, with 630 total worldwide patients, will be run out of Barts Health NHS Trust in conjunction with Queen Mary University of London and will involve a long-acting injection being administered to patients once every six months.
The study is being backed by the National Institute for Health and Care Research (NIHR) and, if successful, could change how adults with high blood pressure are treated forever.
Dr Manish Saxena, study lead and Deputy Clinical Director at Queen Mary University of London, Hypertension Specialist at Barts Health NHS Trust said: “We are excited to be trialling this first of its kind approach to research if it is safe and effective for the treatment of high blood pressure."
He added: “Solving health challenges on this scale cannot be achieved by one person or entity alone. We are thrilled to be working alongside [partners] and combining our expertise to hopefully change modern medicine.”
Approximately a third of adults in the UK suffer from high blood pressure, with key risk factors including being overweight, a poor diet with excess salt and not enough fruit and vegetables, along with smoking and a lack of exercise. If left untreated high blood pressure can considerably increase the risk of heart attacks and strokes.
The drug the scientists will be using is called Zilebesiran, which is an investigational RNA interference (RNAi) therapeutic targeting angiotensinogen (AGT) – a protein produced by the liver and involved in regulating blood pressure. Zilebesiran is administered under the skin and is designed to inhibit the production of AGT preventing constriction of blood vessels which may help reduce elevated blood pressure.
Half of the people with high blood pressure in the UK aren’t diagnosed or receiving treatment, therefore providing a more diverse range of treatments will be beneficial to the population.
For more information on the study, click here.