The Medicines and Healthcare products Regulatory Agency has launched a new monthly safety bulletin, the ‘MHRA Safety Roundup’, as part of its three-year Strategy for Improving Safety Communications.
This initiative aims to make information about medicines and medical devices clearer and more accessible for healthcare professionals.
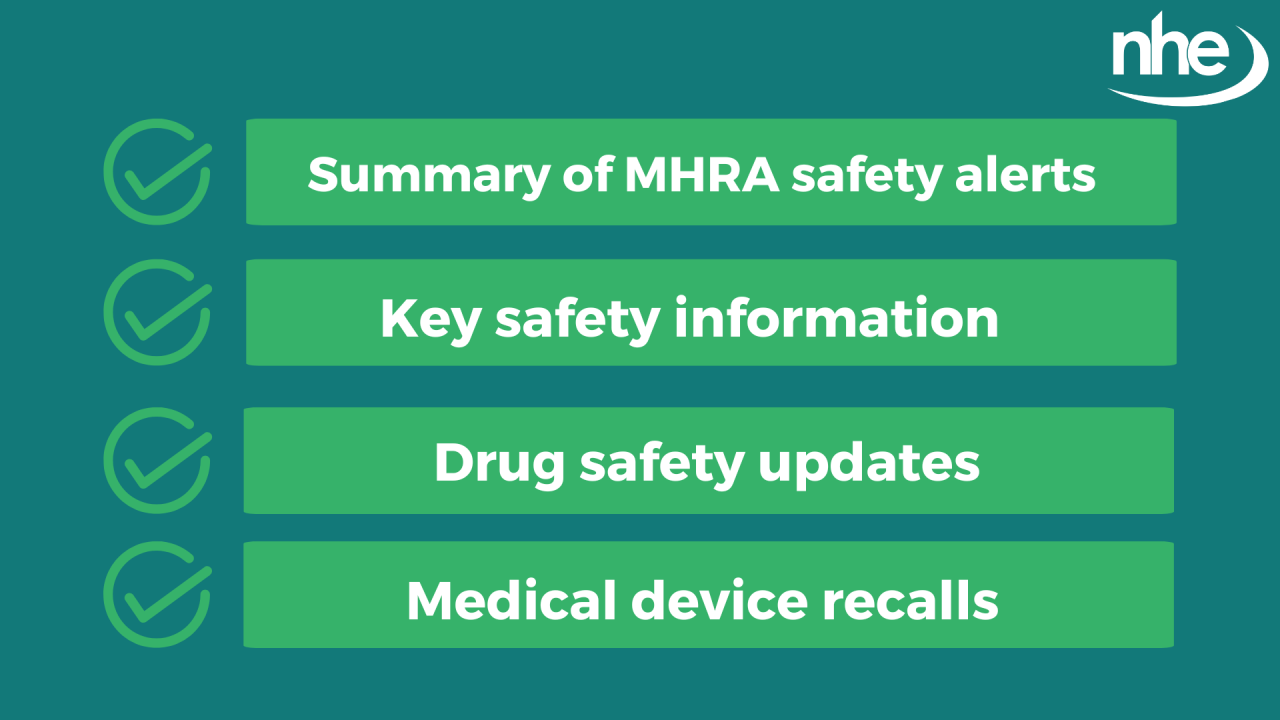
The bulletin, sent to subscribers and published online at the end of each month, provides a summary of all MHRA safety alerts for the past month. This includes drug safety updates (DSU), device safety information (DSI), national patient safety alerts, recalls, medicines notifications, and letters sent to healthcare professionals.
The ‘MHRA Safety Roundup’ also features a news section highlighting key safety information about medicines, medical devices, and healthcare products that may be of interest to readers.
The creation of the bulletin responds to findings from a consultation on how the MHRA communicates safety information. Healthcare professionals, including GPs, nurses, and pharmacists, found it useful to receive information at different frequencies, including monthly summaries.
As part of the strategy’s first-year goals, the MHRA has redesigned all safety alerts to make critical safety advice clearer and easier to action. This includes using colour and relevant imagery to better engage healthcare professionals.
The MHRA continues to improve safety communications, with the next focus on strengthening engagement with patients and the wider public through tailored communication methods.
Image credit: iStock